'Boiling Away' Questions
about Molecular Components
of Earth's Atmosphere
(unanswered by physicists?)
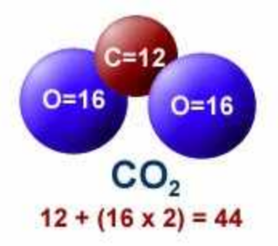
Atomic weights rounded to integers.
C = 12
O = 16
CO2 = 44
N2 = 28
O2 = 32
'Boiling Away' Questionsabout Molecular Componentsof Earth's Atmosphere
|

Atomic weights rounded to integers. C = 12 O = 16 CO2 = 44 N2 = 28 O2 = 32 |
(2017 Feb blog post)
Home >
Blog menu >
This page posing questions on
'boiling away' of atmospheric gas molecules
(lighter ones first? --- N2 and O2 before CO2?)
! Note !
Paragraphs or web-links or images may be added
(or changed), if/when I re-visit this page.
|
INTRODUCTION : With all the talk about
I am motivated to ask some questions about the dynamics of the concentration levels of molecules of various gases in the Earth's atmosphere --- especially in relation to their possible 'escape' from the atmosphere --- an apparent 'boiling off'. Since I have long been interested in math and physics (and mathematical physics), I am prone to thinking about the atmosphere as being this huge, on-going, raucous, 3-dimensional 'billiard balls game' ---- of molecules of various weights crashing into each other --- and rebounding with higher or lower velocities, related to their relative weights (or masses). At the same time, the Earth's gravitational attraction is 'hugging' those atmospheric gas molecules close to the surface of the Earth. And the molecules are getting their energy from a complex mix of various kinds of thermal energy transfer, including
In this scenario, no matter the complexity of the energy transfer process, it seems that the heavier molecules could dominate over the lighter molecules, in the sense that the lighter molecules, when they are headed away from Earth, could be more likely to exit the Earth's 'sphere of influence'. The lighter molecules could be more likely to head for outer space (or the moon's gravitational field --- or the sun's gravitational field --- or some planet's gravitational field) --- for example, whenever a molecular collision causes a molecule to exceed 'escape velocity' --- and the molecule manages to avoid a collision 'on its way out'. Constituents of the atmosphere In thinking about these issues, it helps to consider the molecular composition of the atmosphere and the percentages of those components. A quick web search reveals that: By volume, dry air contains 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere. However, to assure that this does not give a skewed view of the components of the atmosphere, it seems advisable to ask for the composition by weight. A WEB SEARCH on keywords such as 'nitrogen oxygen carbon dioxide percentage in the atmosphere "by weight"' yields sites such as EngineeringToolbox.com, which shows the percentages by weight. Here is a re-ordering of the table there --- with the largest percentages listed first. |
Gas ------ Percent ------ Molecular Chemical Boiling
Molecule By volume By weight Mass Symbol Point (C)
--------- --------- --------- -------- ---------
Nitrogen 78.09 75.47 28.02 N2 -195.79
Oxygen 20.95 23.20 32.00 O2 -182.95
Argon 0.933 1.28 39.94 Ar -186.
Carbon Dioxide 0.03 0.046 44.01 CO2 -78.5
Neon 0.0018 0.0012 20.18 Ne -246.
Helium 0.0005 0.00007 4.00 He -269.
Krypton 0.0001 0.0003 83.8 Kr -153.4
Hydrogen 0.00005 ~ 0 2.02 H2 -252.87
Xenon 9 10-6 0.000044 131.29 Xe -108.1
|
Note that the percents result in almost the same sort order, whether we use volume or weight percents.
NOTE: Other molecules, such as CO2, comprise less than one percent. The average amount of water vapor in the air (0.4% to 1%) is about 10 to 25 times more than the amount of CO2 (0.04%). The ratio of N2-and-O2 to CO2 is about 99.04 to 0.04 --- about 2,476 times more N2-and-O2 than CO2. (This raises questions that have been bugging me: Have scientists like Bill Nye, the 'science guy', been over-emphasizing the infrared radiation absorbing effects of CO2 compared to N2-and-O2? Specifically: Is the relatively small amount of CO2 REALLY many, many times more infrared absorptive than the relatively huge amount N2-and-O2 in the atmosphere? In particular, do the relatively large amounts of N2-and-O2 (and water vapor) in the air make for more of a 'green house' effect than the relatively small amount of CO2? And have U.S. oil companies been getting many taxpayer dollars to build huge CO2 'sequestration' plants that they use to force still more fossil fuels from the ground --- which, when burned, contribute more CO2 to what they are sequestering? These are questions for another blog page.) On 'escape' and relative molecular weights If the conjecture that the lighter molecules might be more likely to 'escape' from the Earth (i.e. in greater numbers per unit of time) is true, then it looks like Nitrogen molecules (according to the 'Molecular Mass' column) might be 'the first to go' --- among the 'top 4'. Oxygen might be the next most 'likely to escape' --- with Carbon Dioxide being the least likely of the 'top 4'. Would there be a (very slow) 'escaping' of nitrogen and oxygen --- relative to heavier CO2 and Argon? Could this tend to favor a (very slow) relative-build-up of Carbon Dioxide? --- even if there were not the (very fast) factors of fossil-fuel burning and the destruction of CO2-consuming plant life adding to CO2 build-up? Futhermore, the very light hydrogen and helium molecules (in the upper atmosphere, unhindered by further collisions) might be REALLY likely to be accelerated to escape velocity --- resulting in 'atmospheric (or Jeans) escape'. --- After getting a handle on the molecular composition of the atmosphere, it then becomes a question of what is the (relative) mass of the 'molecules of interest' --- in order to support a quantitative study of their dynamics ... and some 'molecules of interest' may not be in the brief table above. Here is one table of molecular weights --- with some 'molecules of interest' marked with a red dot. If one were concerned with the dynamics of the petroleum-related and man-made-pollution-related molecules, one might be interested in methane, benzene, ethylene, ethane, propane, etc. --- and ozone. |
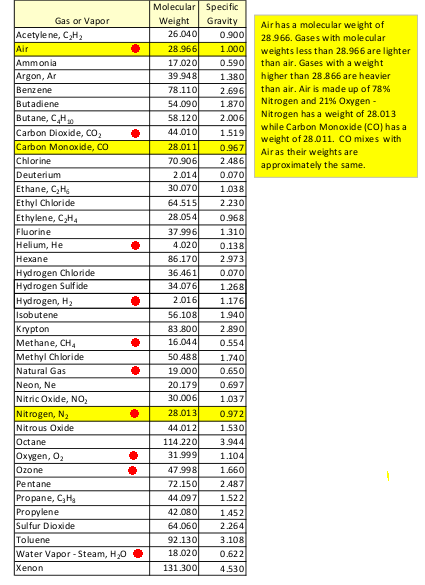
I have marked hydrogen (H2) and helium, because
they are the most common molecules in the known universe.
I have marked methane (CH4) and 'natural gas',
because they are often the subject of localized or
large-volume pollution concerns.
I have marked ozone (O3), because it is often
the subject of ultra-violet shielding concerns.
|
Here is a re-ordering of part of that table, with the heavier molecules ('billiard balls') shown first. |
Molecular
Gas or Vapor Weight
---------------------- -------------
Ozone, O3 47.998
Carbon Dioxide, CO2 44.010 *4
Argon, Ar 39.948 *3
Oxygen, O2 31.999 *2
Air (mixture) 28.966 (average)
Nitrogen, N2 28.013 *1
Carbon Monoxide, CO 28.011
Natural Gas 19.00
WaterVapor/Steam, H2O 18.020
Methane, CH4 16.044
Helium, He 4.020
Hydrogen, H2 2.016
|
I have indicated (with asterisks) the 4 most copious molecular components in Earth's atmosphere. It looks like Ozone might be less likely to 'escape' --- but it might be depleted by chemical means. It looks like Methane and Natural Gas (being lighter than Argon, Oxygen, Nitrogen, and Carbon Dioxide) might be more likely to be 'forced to escape' --- by collisions with heavier molecules.
How to best simulate Earth's atmospheric Astrophysicists have been using super-computers for many years now (now = 2017) to simulate the motion of celestial bodies such as the hundreds of thousands of bodies in a spiral galaxy --- via N-body simulations. And, using the inverse-square-law of gravitational attraction and systems of differential equations describing the forces between all the bodies, they have been performing simulations of how a huge mass of separate particles/bodies distributed in 3-dimensional space might evolve into a planetary system such as our solar system. And, on an even grander scale, they have modeled how millions of bodies distributed in a model of our early universe might cluster together into galaxies --- and continue to evolve, perhaps to the point of forming 'black holes'. It seems that such methods might be used in simulating a mixture of molecules (distributed in an initial configuration around Earth, and with randomized initial velocities) --- to see how they might evolve --- in particular, to see if certain molecules could acquire velocities exceeding 'escape velocity' --- and to see if the remaining mixture of molecules (percentages) would be changed. Perhaps one could reduce the size of the problem somewhat by considering a 'column' of atmospheric gas, extending above a small patch of the Earth's surface. Simulations of atmospheric molecular dynamics may involve some new considerations in formulating the equations of motion. There would be Earth's gravitational effects on the molecules, but, also, there would need to be modeling of the collisions --- perhaps using algebraic methods --- combined with differential equation methods for modeling the gravitational effects. A hint of the algebraic methods that might be employed is indicated by the treatment of a 1-D Collision of 2 Rigid Masses - a numerical (and graphical) simulation. It seems that one could get a 'small-sized' start on simulating the problem, by considering a few hundred molecules --- of just 2 species --- say nitrogen and oxygen --- and seeing how that would proceed in a scaled-down environment --- say, an open-topped box or cylinder with a one-directional gravity-like force involved, along with the collisions. In such a simplified simulation, we would be ignoring temperature difference effects (assume temperature remains constant throughout --- a 'spatially constant' randomization of the initial molecular velocities) and assume that there are not molecule-generating and molecule-consuming effects (as in conservation of mass). We would also assume there are no wind currents --- no 'jet stream' --- no hurricanes, tornadoes, rain storms, etc. We would also assume there are no contributions to the kinetic energy of the molecules from radiation like solar radiation. We would (at least initially) want to make such assumptions to make the mathematical modelling less complex. The deeper one gets into taking into account the main contributors to the molecular dynamics of the atmosphere, the more one realizes that it is a hugely complex problem, such that any simulation would come into question on the types of assumptions being made. There would be questions on what influences are being left out of consideration (that should be included), as well as questions on whether influences that are included are being modelled properly. An additional complication in modelling the entire atmosphere is the situation that the density of the atmosphere varies with altitude (and temperature influences). Taking those factors into account makes the construction of useful mathematical simulations of atmospheric dynamics even more challenging. A sequence of more and more 'involved' simulations might be a productive approach to take. A 'macro' modelling approach may be better? To avoid the massive numbers of equations (differential and algebraic) to be 'integrated', there may be a way to get some answers by using the methods of 'statistical mechanics'. I have done almost no study of statistical mechanics, so I do not know (at this time) how to proceed using those methods. --- There may be a way to get some answers by proceeding on a 'macro' level --- using pressure, density, volume considerations --- and not going down to the level of simulating individual molecular trajectories. --- At this point, I am about out of ideas on the best way to proceed. I retire until another day --- when I might have more to add on these matters. CONCLUSION : There may be no conclusion to this web page, unless I find a comprehensive treatment of 'atmospheric evolution' questions, for Mother Earth. If I find no such super-answer, I hope to continue adding observations and notes and links to this page --- until my ashes are spread to mix with the atoms of the universe. |
|
FOR FURTHER INFORMATION : For further information on atmosphere-dynamics questions, here are some links to do WEB SEARCHES on keywords related to this topic. After the search window appears with an intial page of 'hits', you can change or add keywords to hone the search to what you are looking for. In 2017 Feb, I see many 'hits' that might answer some of my questions or, at least, give me some more information that should be added to this page. I may be examining 'hits' from these searches as time permits. |
|
Bottom of this page on blog topic
To return to a previously visited web page location, click on
the Back button of your web browser, a sufficient number of times.
OR, use the History-list option of your web browser.
< Go to Top of Page, above. >Or you can scroll up, to the top of this page. Page history:
Page was posted 2017 Feb 21.
|